遗传性听觉损失重大突破,基因编辑可预防老鼠耳聋 [英国媒体]
(科学家们)首次使用基因编辑技术预防了老鼠产生耳聋,这可能会改变未来遗传性耳聋的治疗方式。英国网友:“实际上有一种解决方法:缴纳斯堪的纳维亚式(北欧式)的税以获得斯堪的纳维亚式的教育和社会服务。所以(我们需要的是)25%的增值税,30%的基本税率(不包含个人精贴)以及50%的最高税率。”
-------------译者:*慢活族*-审核者:龙腾翻译总管------------
Deafness has been prevented in mice using gene editing for the first time in an advance that could transform future treatment of genetic hearing loss.
(科学家们)首次使用基因编辑技术预防了老鼠产生耳聋,这可能会改变未来遗传性耳聋的治疗方式。
The study found that a single injection of a gene editing cocktail prevented progressive deafness in baby animals that were destined to lose their hearing.
研究发现,单次注入一种基因编辑混合液可预防那些遗传到渐进性耳聋基因注定失去听力的小动物们(患上耳聋)。
“We hope that the work will one day inform the development of a cure for certain forms of genetic deafness in people” said Prof David Liu who led the work at Harvard University and MIT.
在哈佛大学及麻省理工学院领导这项工作的教授David Liu说:“我们希望有朝一日这项工作能为人类治疗某些形式的遗传性耳聋提供帮助。”
Nearly half of all cases of deafness have a genetic root but current treatment options are limited. However the advent of new high-precision gene editing tools such as Crispr has raised the prospect of a new class of therapies that target the underlying problem.
近一半的耳聋病例都有遗传因素在内,但目前的治疗方式很有限。然而,CRISPR(注1)等新型高精度基因编辑技术的出现,提高了这类针对深层(遗传)问题的新疗法的应用前景。
The study published in the journal Nature focused on a mutation in a gene called Tmc1 a single wrong letter in the genetic code that causes the loss of the inner ear’s hair cells over time.
《自然》期刊上刊载的这项研究集中于发生在名为TMC1的基因里的一个突变——即遗传密码中错误的一个碱基(可能为鸟嘌呤、腺嘌呤、胞嘧啶和胸腺嘧啶),随着时间的推移将导致内耳毛细胞损伤(注2)。
注1:CRISPR:规律成簇的间隔短回文重复(Clustered Regularly Interspaced Short Palindromic Repeats),是大多数细菌及古细菌的一种获得性免疫方式。现在使用的CRISPR/cas 9基因编辑技术是由单链的引物 RNA和有核酸内切酶活性的Cas 9蛋白构成。通过cas9蛋白导致DNA双链的断裂,而细胞通过NHEJ的修复造成INDEL效应(insertion and deletion),进而造成基因的移码突变从而达到基因敲除的目的。
注2:TMC1发生了一种显性负性错义突变后,会导致内耳毛细胞单通道电流水平和钙渗透率降低,进一步导致感音神经性语后聋(语言形成后出现的耳聋)。通常,TMC1显性突变患者在10-15岁时会开始逐渐变聋。
附注:TMC1基因综述:http://www.doc88.com/p-6651998885876.html
-------------译者:*慢活族*-审核者:龙腾翻译总管------------
The delicate hairs which sit in a spiral-shaped organ called the cochlea vibrate in response to sound waves. Nerve cells pick up the physical motion and transmit it to the brain where it is perceived as sound.
这种纤细的茸毛位于名为耳蜗的螺旋状器官中,该器官可以响应声波而振动。神经细胞会接收其物理运动(信号)并将其传送至大脑,在那里它被理解成声音。
If a child inherits one copy of the mutated Tmc1 gene they will suffer progressive hearing loss normally starting in the first decade of life and resulting in profound deafness within 10 to 15 years. However since most people affected by the mutation will also have a healthy version of the gene inherited from their other parent the scientists wanted to explore whether deleting the faulty version worked as a treatment.
如果有子女继承了突变TMC1基因的一份拷贝,他们的听力将逐渐遭受损失,通常从出生后的第10年开始发病,在10至15年之内将患上严重的耳聋。然而,由于受到突变影响的大多数患者也会从其父母那儿遗传到一份正常健全的基因拷贝,科学家们便设想研究是否能够通过删除有缺陷的基因拷贝来达到治疗目的。
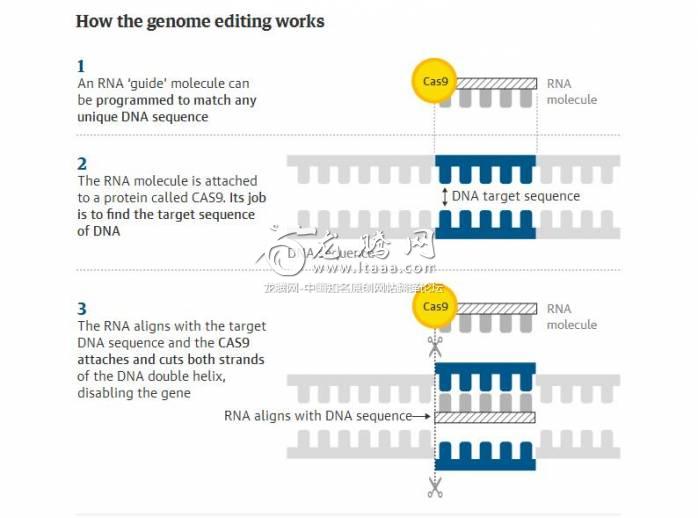
(图解:如何实现基因编辑:
1、一个RNA(核糖体)“引物”分子程序性地结合到其特定的DNA序列上;
2、RNA分子连接一个名为CAS9的蛋白,该蛋白的作用为发现目标DNA序列;
3、将RNA与目标DNA序列排成一条线,CAS9连接并切断DNA的两条双螺旋链,使基因失活。)
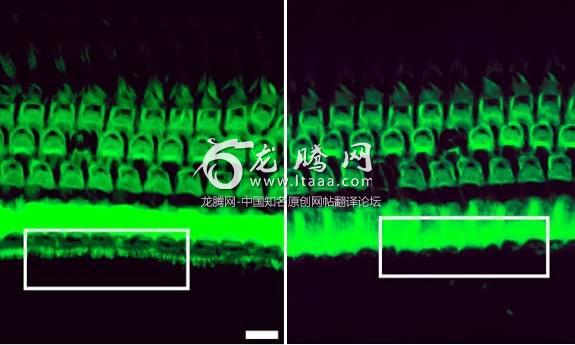
Liu and colleagues used gene editing technology known as Crispr-Cas9 which acts as a molecular scissors snipping the genome to disable a target gene. The team injected the gene editing solution into the inner ears of baby mice with the hearing loss mutation. After eight weeks hair cells in treated ears resembled those in healthy animals – densely packed and tufted with hairlike bundles. The hair cells of untreated mice in contrast looked damaged and sparse.
刘教授和他的同事们使用了被称为CRISPR-Cas9的基因编辑技术,这种技术可以作为分子剪刀剪切基因组,从而使目标基因失效。研究小组将基因编辑溶液注入到含有这种听力损伤突变基因的幼鼠的内耳中。八周后,治疗后的耳内毛细胞就像健康的动物一样——密密麻麻,茸毛丛生。相比之下,未经治疗的老鼠的毛细胞看起来像是受过损伤似的,稀稀疏疏。
-------------译者:*慢活族*-审核者:龙腾翻译总管------------
Then the researchers conducted a hearing test on the mice by placing electrodes on their heads and monitoring the activity of brain regions involved in hearing. Researchers needed more sound to spark brain activity in untreated mice compared with treated mice the team found. On average after four weeks treated ears could hear sounds about 15 decibels lower than untreated ears. “That’s roughly the difference between a quiet conversation and a garbage disposal” Liu said.
接着,研究人员将电极放置在老鼠头部,监测参与听觉形成的大脑区域的活动,对老鼠进行听觉测试。该小组发现,与经过治疗的老鼠相比,研究人员需要给予更大的声音刺激未经治疗的小鼠才会产生应有的脑部活动。平均来说,四个星期后,经过治疗的耳朵能比未经治疗的耳朵听到低15分贝的声音。刘教授说:“这大体上相当于说悄悄话和清理垃圾之间发出的声音大小的差异”。
Simon Waddington a reader in gene transfer technology at University College London described the study as an elegant application of new gene editing tools. “Hitherto incurable and often even untreatable diseases are now within the scope of gene therapy” he said.
伦敦大学学院基因转移技术专家西蒙·沃丁顿,把这项研究描述为基因编辑工具的一次优雅的全新应用。他说:“迄今为止无法治愈、甚至时常无法治疗的那些疾病现在都被纳入了基因治疗的范畴。”
The team plans to develop the therapy in larger animals to ensure the method is safe and effective before moving closer to a patient trial.
该小组正计划在更大的动物身上应用这种疗法,以确保再进一步给患者试验之前验证这种方法是安全有效的。
Previously the option to carry out screening for genetic causes of deafness during IVF treatments has prompted an ethical debate with some deaf couples seeking to use screening to sext embryos carrying the deafness gene. In the UK this was banned under legislation introduced in 2008. Liu added: “We also recognise the importance and remain mindful of cultural considerations within the deaf community as this work moves forward.”
此前,在试管受精治疗中,由于一些聋人夫妇试图通过筛选挑出携带致聋基因的胚胎,所以是否能根据诱发耳聋的遗传因素进行筛选引起了伦理学上的一场争论。英国于2008颁布的法案禁止了该项筛查。刘教授补充道:“我们也认识到聋人群体的重要性,在这项工作向前推进的同时,也会详细考虑聋人群体的相关文化。”
-------------译者:风起云团-审核者:龙腾翻译总管------------
bllckchps 8h ago
Amazing. Everything probably sounds a little crispr.
太棒了。这一切听起来有点crispr的感觉。
bpollutin bllckchps 6h ago
Great news...we all need some in today's America
不错的消息...这正是现今我们美国需要的。
MehHem bllckchps 4h ago
Hear hear!
说得对!说得对!
OldEnglishMarmalade 8h ago
Fantastic news.
美妙的新闻。
Is it too late to correct the genomes of members of the current government all stone deaf to the problems of homelessness and of underfunded health and education systems?
对于那些在流浪汉、公共卫生和教育系统问题上完全失聪的现任政府来说,现在纠正他们的基因组是否为时已晚?
profangus OldEnglishMarmalade 7h ago
That condition is known as sextive deafness.
这种情况通常称之为选择性耳聋。
CaptainGrey OldEnglishMarmalade 6h ago
Meanwhile your fellow travellers in the financially illiterate brigade today make a plea for drugs to be reduced to £1 so stopping all such research.
与此同时,跟你们一伙的金融文盲们正在请求把毒品降到1英镑,所以还是停下这些研究吧。
Actually there is a solution: paying Scandinavian levels of tax to have Scandinavian levels of education/social services. So VAT 25% basic rate 30% (no personal allowance) and top rate 50%. But that would mean Guardianistas paying more when we all know that taxes are for people "richer than me".
实际上有一种解决方法:缴纳斯堪的纳维亚式(北欧式)的税以获得斯堪的纳维亚式的教育和社会服务。所以(我们需要的是)25%的增值税,30%的基本税率(不包含个人精贴)以及50%的最高税率。但那只意味着中产阶级(Guardianistas:卫报读者,多为中产阶级)要付出更多,我们都知道我们纳的税都用来服务那些“比我富有的”人们。
So would you be happy with a big tax rise for yourself? That is the $64000 question.
所以你会为自己的赋税增加而感到高兴吗?这可是个价值6.4万美元的问题。
My guess is "no" but I hope to be be pleasantly surprised.
我觉得你的答案是“不”,但我期待你能让我惊讶。
Anbaric CaptainGrey 6h ago
“Actually there is a solution: paying Scandinavian levels of tax to have Scandinavian levels of education/social services. So VAT 25% basic rate 30% (no personal allowance) and top rate 50%.”
“实际上有一种解决方法:缴纳斯堪的纳维亚式(北欧式)的税以获得斯堪的纳维亚式的教育和社会服务。所以(我们需要的是)25%的增值税,30%的基本税率(不包含个人精贴)以及50%的最高税率。”
Sounds good to me.
我觉着这听起来不错啊。
JazzHorse 8h ago
Crispr sounds like a dating app for crisps.
Also this is good news. Well done everyone.
Crispr听起来像是薯片用的约炮软件。
不过新闻倒是个好消息。干得好伙计们。
Brian Davies 5h ago
I suffer with meniers disease I have permanent tinnitus and the vertigo quite bad the end of this is deafness.. I don't wish to lose it so hopefully some kind of cure will be found
我患有美尼尔病(注:因膜迷路积水引起阵发性眩晕、耳聋、耳鸣),我有永久性耳鸣和严重的眩晕,最后(我的病)将发展为耳聋...我并不想失去听力所以很希望能够找到一些治愈的方法。
版权声明
我们致力于传递世界各地老百姓最真实、最直接、最详尽的对中国的看法
【版权与免责声明】如发现内容存在版权问题,烦请提供相关信息发邮件,
我们将及时沟通与处理。本站内容除非来源注明五毛网,否则均为网友转载,涉及言论、版权与本站无关。
本文仅代表作者观点,不代表本站立场。
本文来自网络,如有侵权及时联系本网站。
图文文章RECOMMEND
热门文章HOT NEWS
-
1
最近,新冠肺炎疫情在日本有扩大的趋势,有专家呼吁日本应当举国行动起来,共...
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
推荐文章HOT NEWS
-
1
最近,新冠肺炎疫情在日本有扩大的趋势,有专家呼吁日本应当举国行动起来,共...
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10